04 Mar New Pharmacotoxicology Data Linked to the Pre-Clinical Studies
Posted at 10:08h
in Pll Therapeutics
SUMMARY OF EFFECT ON MUSCLE WASTE AND NEUROTOXICITY
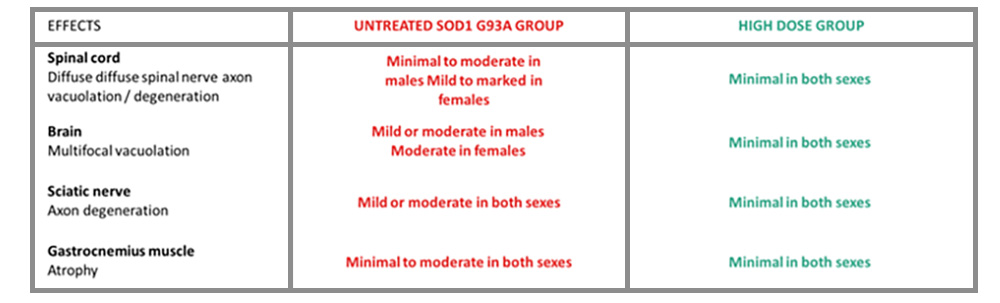
Transgenic G93A-SOD1 mice that received the hightest TC-X dosage showed a much-reduced incidence and severity of nervous system and skeletal muscle pathology when compared to control and low dose mice. CR Conclusions Charles River

